Sub-research Introduction
Sub A: [Fuel Cells]
Among fuel cells that generate electricity with hydrogen and oxygen, polymer electrolyte fuel cells (PEFCs) are required to achieve higher efficiency and lower cost as a driving source for automobiles. To improve the performance of PEFCs, it is important to reduce and sophisticate the materials used such as platinum catalysts and electrolyte membranes. In addition, improving the output as a battery is also extremely important; in particular, increasing the current density, i.e., improving the reaction rate at the electrode interface, is indispensable. In particular, the key is how quickly and uniformly the reactive species (electrons, protons, oxygen, or hydrogen) are delivered to the 2-5 nm Pt catalyst surface to improve the reaction rate. Among these approaches, this research group focuses on the catalyst layer. The catalyst layer generally consists of carbon black (particle size 20-50 nm) as the backbone, an electrolyte (ionomer), and voids with a pore size of approximately 100 nm, through which electrons, protons, and reaction gases are transported in each phase. Although we know empirically that this complex microstructure has a significant impact on the transport rate of the above materials, some questions remain unanswered, including “What are its main factors?”, “What kind of structure is ideal?”, “Is there room for improvement of the current structure?”, and “What shape of material must be fabricated?”. Therefore, this research group is conducting the following studies to address these questions.
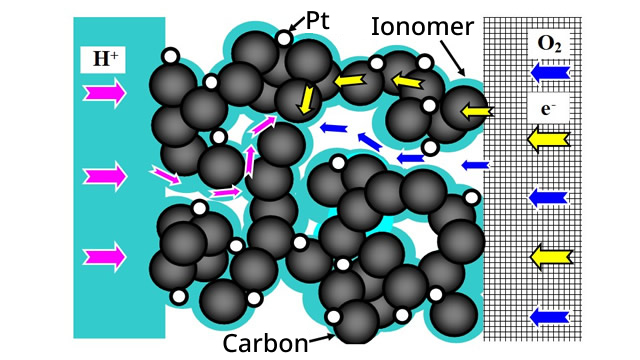
Fig. Schematic of PEFC
Characterization of the microstructure of polymer electrolyte fuel cells
We elucidate the microstructure of an actual catalyst layer with pore sizes ranging from several 10 to 100 nm using advanced measurement equipment such as a focused ion beam scanning electron microscope (FIB-SEM) and nano/micro X-ray CT. In addition, by evaluating the transport characteristics using numerical reconstruction and comparing theoretical models, we clarify the issues in the current catalyst layer and propose guidelines for further performance improvement. In particular, this method is characterized by qualitative and quantitative verification of the influence of the microstructure through comparisons employing experimental measurements, numerical predictions, theoretical evaluations, and model structures.
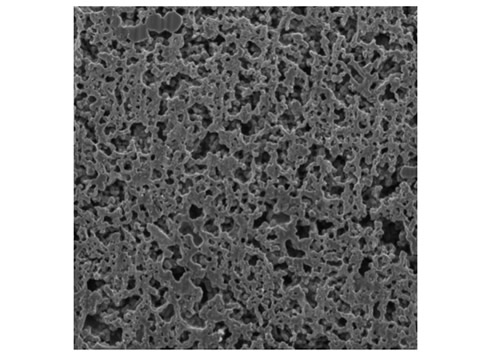
Fig. The Structure of PEFC Catalyst Layer (Cross-sectional Image)
Reaction transport simulation of polymer electrolyte fuel cells
We are developing a large-scale simulator that couples electrochemical reactions and mass transport phenomena in the fuel cell catalyst layer, which are difficult to measure directly. In addition to calculations for actual complex microstructures, we have modeled the aggregate structure of carbon black, the electrolyte coating structure, and platinum catalyst distribution and verified their effects through numerical simulations. In particular, the model is a multi-scale, multi-phase, multi-component analytical model, and the calculations incorporate the properties of actual materials. We also perform a two-phase flow analysis of liquid-water transport and phase changes.
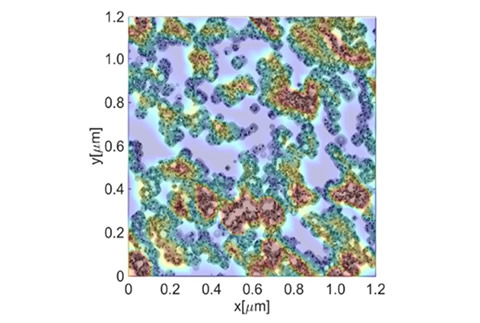
Fig. Calculation Example for Reaction Distribution in the Catalyst Layer
Catalyst layer structure design for various novel materials for polymer electrolyte fuel cells
Although research and development of new catalysts and electrolytes is being actively performed domestically and internationally, evaluation of their battery properties is conducted in line with the structure and conditions optimized for conventional materials. Therefore, the structure and conditions are not necessarily suited to the unique properties of each material. In this study, we are collaborating with research groups in electro-catalyst and electrolyte material development to clarify the ideal structure by numerical analysis and theory, considering their physical properties and realizing the structure by applying various fabrication processes, such as the ink-jet method.

Fig. Automatic Film Applicator
Multiscale simulation for polymer electrolyte fuel cells
Besides reaction transport analysis in the catalyst layer, we are also conducting multi-phase, multi-component reaction transport multi-scale simulations in flow paths, cells, and stacks. We are currently working on research related to overall system design and stable control through this.
Fig. Various Simulation Technologies for PEFCs
Sub B: [Lithium-ion Batteries & Next-generation Rechargeable Batteries]
There is a growing demand for higher-performance lithium-ion batteries (LiBs) as a drive source for electric and hybrid vehicles. In particular, for automotive applications, it is important to achieve high capacity and high output, which are performance requirements related to cruising range and charging speed. LiBs have a porous structure with active material particles usually deposited to mutually transport and store lithium ions between the cathode and anode during charging and discharging. The key to achieving high capacity and high output is to increase the active material packing density while ensuring the connectivity of the voids through which the electrolytic solution penetrates and serves as the ion conduction pathway. Furthermore, it is necessary to achieve the transfer of electrons as well as ions. Reaction and transport phenomena within this microstructure are still largely unknown, and it is unclear whether conventional empirical fabrication methods have reached the theoretical performance, which is the physical limit. Therefore, this research group is conducting the following studies.
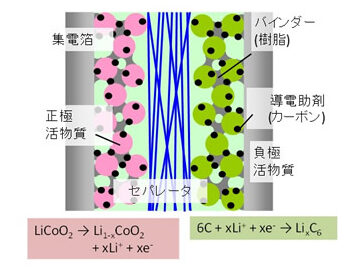
Fig. Schematic of LiB
Elucidation of internal phenomena of lithium-ion batteries
We are conducting reaction transport simulations with respect to the structure of actual LiBs. We elucidate the actual electrode structure using advanced measuring devices such as focused ion beam scanning electron microscopy (FIB-SEM) and nano/micro X-ray CT. In addition, we compare numerical predictions of effective ionic conductivity with electrochemical measurements, clarified the differences from theoretical models, and propose guidelines for improving the electrode structure. We are also conducting joint research on Li concentration distribution measurements during actual charging and discharging to validate the calculation model and construct a theoretical model. Through the above studies, we are developing supporting technologies for LiB electrode structure design.
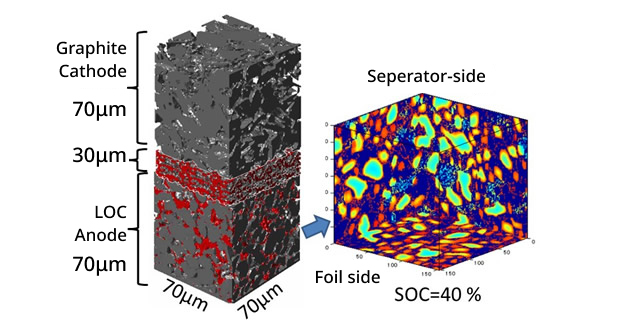
Fig. LiB Simulation Example
Electrode structure design using machine learning
For the optimal design of porous structures in electrochemical devices, it is necessary to maximize evaluation functions such as capacitance and power output by comprehensively considering many requirements, such as increasing the effective reaction field area, improving the effective ionic conductivity, achieving uniform current density distribution for improved durability, maintaining the transport balance between electrons and ions, and suppressing side reactions. The optimum conditions must be determined each time the physical properties of the material used or the particle size distribution changes. To solve such complex problems, we utilize numerical simulation techniques to computationally solve optimal value problems. Currently, we are developing an automated design technique for electrode structure conditions using genetic algorithms. Through the above studies, we aim to shorten the time-consuming search for the optimal structure, which has been performed empirically in the past.
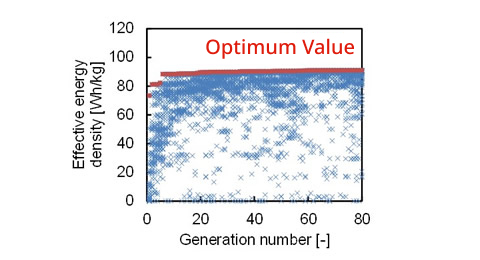
Fig. Genetic Algorithm Calculation Example
Analysis of reaction transport within the 3D electrode structure
A normal LiB has a structure in which a cathode composite layer, a separator, and an anode composite layer, all having a layered structure, are sandwiched. However, as the capacity increases, the film becomes thicker and ion conduction to the deeper electrode layers becomes insufficient, leading to a decrease in electrode utilization. To overcome this issue, we are also working on making the electrode structure three-dimensional (3D). Although 3D conversion is advantageous for securing the ion conduction field area, fabricating such structures has been difficult in the past. Therefore, we utilize inkjet coating equipment and structural design using numerical analysis technology.
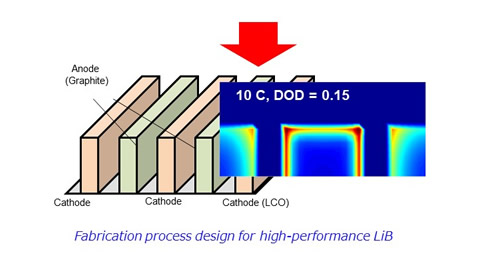
Fig The Example of 3D Electrode Structure Calculation.
Application to next-generation rechargeable batteries
From the viewpoint of further increasing the capacity, durability, and safety of LiBs, developing next-generation batteries, such as all-solid-state batteries and air batteries, is being actively pursued. This research group also uses numerical analysis methods to design the structure of these new batteries and identify issues that may arise when using the new materials.
Other research related to porous media and mass transfer
We are expanding the applications of various measurement and evaluation technologies, such as FIB-SEM and X-ray CT, for numerical simulation of coupled porous structures, electrochemical reactions, and mass transport in fuel cells and secondary batteries to other fields. With the motto of actively fusing various fields, we also promote collaboration with researchers and engineers from industry and academia in Japan and abroad.
Examples: various separation membranes, other electrochemical devices, catalytic reactors


